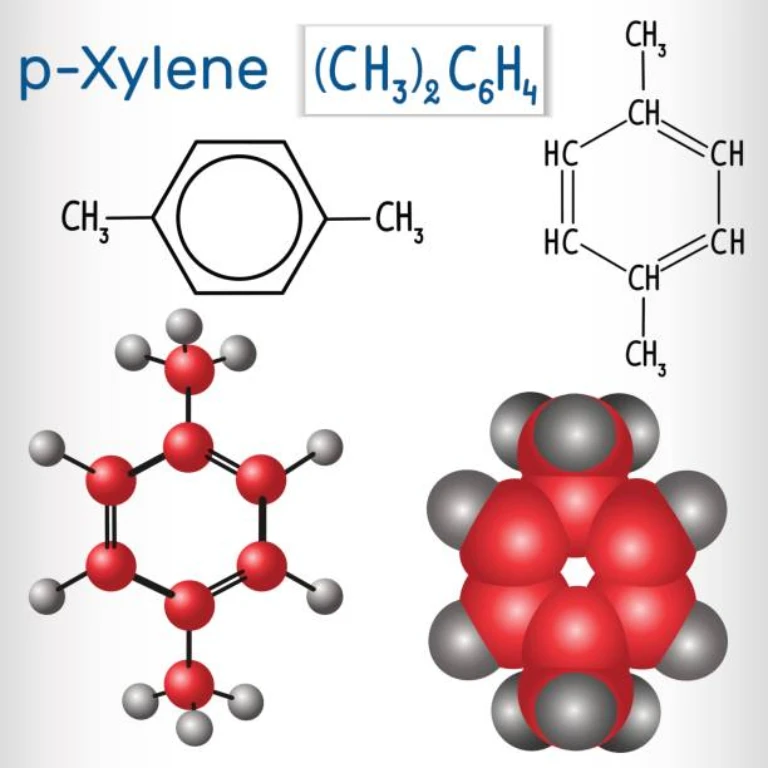
Introduction to p-Xylene: Properties and Significance
p-Xylene. It may sound like jargon, a term that is only used in the laboratories and factories of chemical engineers. But if one looks at it in a broader perspective, it is a colourless liquid hydrocarbon that has become an essential part in today’s world and is a key constituent of the petrochemical industry. It is not just the equation written on the whiteboard but it is the unseen force behind the synthesis of terephthalic acid of numerous products that we use in our daily lives, from the fabric we wear to the bottles that contain our beverages.
In fact, p-xylene is one of the three xylene isomers that are categorized based on the position of the methyl groups on the benzene ring. This small difference in structure brings out a lot of differences in characteristics, and p-xylene is the most valuable among all the siblings. Its importance arises from the fact that it is the principal feedstock in the production of terephthalic acid (PTA) manufacture, which is the basic ingredient of PET. PET, in turn, is the plastic used in bottles for soft drinks and water, synthetic fibers in our clothes, and countless protective and delivery packaging for the products we consume. To understand p-xylene is to understand an element that is used in the manufacturing process of most products in modern society. It is a part of the contemporary world that is not easily visible, but without it, people cannot make various objects.
This is your guide to p-Xylene – its chemical composition, uses, and the innovations that are defining its development. We will look at how this molecule is formed, how it is used, and the market factors that regulate its industry.
Raw Materials and Feedstock for p-Xylene Production
| Feedstock | Source | Main Components | Role in p-Xylene Production |
| Naphtha | Crude oil refining or catalytic cracking | Benzene, toluene, xylenes | Primary feedstock providing necessary aromatics for p-xylene production |
| Toluene | Distillate or conversion of other aromatics | Toluene (C₇H₈) | Converted into p-xylene through disproportionation or methylation |
| Heavy Aromatics | Refining process byproducts | C8+ aromatics | Selective separation recovers p-xylene from heavy aromatic streams |
| Natural Gas | Refining and petrochemical industry | Methane, ethane | Used to produce methanol, which aids in toluene methylation for p-xylene production |
| Other Aromatic Byproducts | Various petrochemical processes | Blend of benzene, toluene, and xylenes | Additional feedstock separated and purified to enhance p-xylene production |
Key Industrial Processes in p-Xylene Production
To convert these raw materials into p-xylene is a well-coordinated series of chemical reactions that is performed through a series of industrial processes. to produce different products. Each process, with its unique technological needs, is carefully engineered to selectively synthesize and separate p-xylene to the high purity and large quantity required by modern industries. Every step in the process of creating aromatic building blocks and purifying p-xylene is crucial and plays a role in the overall cost-effectiveness of the business. The three primary processes that have been widely used in the production of p-xylene are catalytic reforming, toluene disproportionation (TDP), and toluene methylation. Now, let us discuss how each of these methods can be used to produce large amounts of p-xylene with high purity.
Catalytic Reforming
Catalytic reforming is one of the major ways of producing mixed xylenes, including p-xylene. It is similar to the engine room of aromatic production where low-value naphtha is converted into a valuable stream of benzene, toluene, and xylenes (BTX). This is not just a change in molecules; it is the creation of the possibility of large-scale p-Xylene production.
Within the reformer, a selected catalyst, which is usually based on platinum, initiates a series of reactions at high temperature and pressure, and in the presence of hydrogen. Dehydrogenation helps in the removal of hydrogen to produce aromatic rings, isomerization alters the molecular structure, and cyclization helps in the conversion of straight-chain hydrocarbons into cyclic aromatics. The outcome is a reformulated naphtha stream containing a higher concentration of BTX, xylene isomers in particular – the feedstock for p-xylene.
Reforming does not directly synthesise p-xylene but prepares the ground for its separation. It is the initial process that forms the aromatic-rich feed that is required for the subsequent purification process. In its absence, large-scale production of p-xylene would not be possible if it were to be produced through the same process. It is the initial step in the process of refining and isolating the product, which is the first step in the process.
However, it should be noted that prior to the reforming process, feedstock drying is a crucial step. Small amounts of water can alter the reaction mechanisms, poison the catalysts, reduce the yields of aromatics, and increase the formation of undesired products. To minimize this risk, the drying agents that are used are activated alumina (Al₂O₃) and molecular sieves that help in the removal of any remaining moisture in the feedstock. Of them, molecular sieves ( 3A, 4A, 5A) are the most commonly used adsorbent because of their high selectivity and efficiency, which can bring the water content down to 0.1ppm. This makes sure that the reforming reactions take place under the best conditions possible, thus increasing the life span of the catalysts and the production of aromatics.
Toluene Disproportionation (TDP)
Toluene Disproportionation (TDP) is a versatile and cost-effective process for making xylenes, especially p-xylene, without upsetting the overall equilibrium of the aromatics market. It is more like a chemical balancing process where methyl groups are shifted from toluene to benzene and xylenes, depending on the market needs. This flexibility makes TDP a useful tool for petrochemical producers, as it enables them to adjust production according to the relative demand for these important aromatics.
At its core, TDP converts two toluene molecules into one benzene molecule and one xylene molecule. This conversion is done over zeolite-based catalysts like H-ZSM-5 at specific temperature and pressure conditions. These catalysts contain the active acidic sites that are required to facilitate the reaction and enable the molecular rearrangement. However, TDP produces a mixture of isomers of xylene which include ortho-xylene, meta-xylene, para-xylene and ethylbenzene and hence there is a need for further purification to obtain the highly valued p-xylene.
To maintain high activity of the catalyst and minimize side reactions, it is necessary to control the moisture level. As little as 1% water can deactivate the catalysts, reduce the rate of disproportionation, and increase the formation of unwanted byproducts. To reduce this risk, the feedstock is treated with molecular sieves (4A, 5A) to remove bulk moisture to enhance the dryness of the feed before it is fed into the reactor. Activated alumina (Al₂O₃) removes the last traces, thus avoiding catalyst contamination and ensuring the high efficiency of the reaction. Molecular sieves are particularly effective because of their selectivity towards water and high thermal stability that increases the life of the catalyst and improves the efficiency of the process.
The strength of TDP is that it is market-oriented. When benzene demand is low, TDP can be ramped up to move excess toluene to higher-value xylenes. On the other hand, if the prices of benzene increase, then the supply can be managed to remain constant. Although TDP is not specifically aimed at p-xylene production, it continues to be a significant source of xylene and plays a role in the chain that ultimately provides high-purity p-xylene for various industries.
Toluene Methylation
Toluene methylation is one of the most direct and efficient methods of producing p-xylene. This is achieved by adding a methyl group (-CH₃) to toluene in a way that favours the production of p-xylene and minimizes energy-consuming separation processes. Toluene methylation is more efficient than other routes that involve the separation of p-xylene from other xylene isomers because it synthesizes p-xylene directly.
The reaction occurs in the presence of a highly selective catalyst, which is typically H-ZSM-5 molecular sieves, which directs the methylation to the para-position of the toluene ring. This para-selectivity is important because it enhances the yield of p-xylene while reducing the formation of other isomers, and also reduces the burden on the downstream separation equipment. Moreover, the use of methanol or dimethyl ether (DME) as a methylating agent is also advantageous in terms of sustainability since these reagents can be derived from natural gas or biomass.
However, the performance of the catalyst is highly sensitive to feedstock quality, especially moisture content. Any amount of water greater than 100 ppm can deactivate the acidic sites of H-ZSM-5 and thus affect the reaction and selectivity towards p-xylene. It also increases coke formation, which in turn increases the rate of catalyst deactivation and regeneration costs.
To avoid such problems, thorough drying is crucial. Molecular sieves (4A, 5A) are capable of reducing the moisture level to below 10 ppm, which is much better than other drying agents. The porous structure of the material is highly selective for water removal and does not allow the degradation of toluene and methanol. Activated alumina is used as the second layer to remove any remaining moisture. This drying process is not a luxury but a necessity for maintaining the stability of the catalyst and achieving the highest possible yield of p-xylene.
Due to the improvements in the selectivity of the catalysts and the improvement of the process, toluene methylation is becoming one of the most important technologies for the production of p-xylene to meet the increasing demand in the global market. This method is expected to be one of the key technologies in the future of p-Xylene production as cost-efficiency and sustainability are the main concerns. It is a targeted chemical strategy, which is directed at the intended product with growing precision.
Distillation to remove ethylbenzene (EB)
While catalytic reforming, toluene disproportionation, and toluene methylation are effective in producing p-xylene but the product is not pure p-xylene. The crude xylene stream contains a mixture of C8 aromatics, including ethylbenzene, o-xylene, and m-xylene, which need to be separated or converted.
The first of these steps of the purification process is distillation, which is mainly aimed at the separation of ethylbenzene (EB) from the C8 aromatic mixture. Ethylbenzene and p-xylene have very close boiling points, and thus, basic distillation is not very effective in the separation of the two. However, superfractionation, an improved distillation technique, further improves this separation by taking advantage of small differences in boiling points.
Superfractionation columns are tall structures in petrochemical plants that are intended to provide the greatest contact between vapor and liquid. These columns have many theoretical plates and high reflux ratios, which enhance the separation of ethylbenzene and p-xylene based on the small difference in boiling points. This method does not provide ultra-high purity, but it helps to decrease the ethylbenzene content and thus the burden on other energy-intensive processes, such as adsorption.
Distillation is used as a preliminary step to make the feedstock less complex before more selective sieving processes are applied. It does not provide a complete separation, but it is useful in the first stage of purification of p-xylene and enhances the efficiency of molecular sieving.

Adsorption Separation
Adsorption separation is the most effective method for the separation of p-xylene from the other isomers, it can be described as a molecular filter that only allows p-xylene to pass through while the other isomers are trapped. This is done using mainly the X-type zeolites including NaX and BaX, which have well-defined pore structures that allow for selective adsorption according to the size and shape of the molecules., which have well-defined pore structures that allow for selective adsorption according to the size and shape of the molecules.
Zeolites are crystalline aluminosilicates that are tailored to selectively adsorb p-xylene, while o-xylene, m-xylene, and EB are either non-adsorbed or weakly adsorbed. This high selectivity makes adsorption the most effective technique for the separation of p-xylene from mixed xylenes.
It usually runs in a continuous mode, and the most commonly used technology is the simulated moving bed (SMB). Think of a carousel of adsorbent beds that are in the adsorption phase, the desorption phase, and the regeneration phase at the same time. The feed mixture passes through the zeolite-packed columns where p-xylene is selectively adsorbed. It is then desorbed using a desorbent, such as toluene or paradiethylbenzene, and recovered in its purified form.
Adsorption is always able to produce p-xylene with purities of more than 99.7%, which is why it is widely used in the industry. It is a highly accurate molecular sorting mechanism that is essential for the production of ultra-pure p-xylene for the growing petrochemical and polyester markets.
C8 Aromatics Isomerization
C8 Aromatics Isomerization is the recycling process in the p-xylene production process. It can be viewed as a process of rearranging the molecules, where the “leftovers” – the ortho-xylene and meta-xylene after p-Xylene extraction – are converted to improve the yield of the target molecule. It is an important step in the optimization of resources and reduction of waste, which is in line with the chemical engineering process.
After the selective adsorption of p-Xylene, the stream that is left behind is not just dumped. It is a valuable stream containing ortho-xylene and meta-xylene, ethylbenzene, and p-Xylene that was not converted. This stream is fed to the isomerization unit. Here, ortho-xylene and meta-xylene are converted into one another through isomerization reactions under controlled catalytic conditions. These reactions, which are catalyzed by specific catalysts, enable the transformation of a part of the ortho- and meta-isomers back into p-xylene. It also restores the equilibrium of xylene isomers and provides a constant feed of p-Xylene precursors for the next stage of separation.
The isomerate stream, with rich in p-Xylene, will not be wasted. Instead, it is returned back to the separation section, usually after it has gone through the process of ethylbenzene stripping. This closed-loop system is one of the key processes in the production of p-xylene in the modern world. It enhances the overall yield of p-Xylene from the initial BTX feedstock, optimizes the process, and minimizes the consumption of fresh feedstock. Isomerization is the chemical recycler, making sure that aromatic molecules are fully utilized, to maximize the yield of p-Xylene.
Recycling
Recycling is not just a step but a philosophy that has been incorporated into the production of modern p-Xylene. The C8 aromatics isomerization process described above is a good example of this commitment towards efficiency and resource utilization. In addition to isomerization, recycling concepts are applied throughout the production process. The feedstocks, solvents, and catalysts are often recycled, and the amount of waste produced is kept to a minimum while the profitability is maximized. In the current world that is being shaped by the need to embrace sustainability, this inherent need for efficiency in the production of p-Xylene puts it in a good standing within the petrochemical industry. Recycling is not an addendum; it is a part of the process, which underlines the company’s environmentally friendly and efficient approach to chemical production.
Why Choose Jalon Adsorbent for p-Xylene Separation?
For p-xylene producers seeking maximum efficiency and purity, Jalon adsorbent is the trusted choice. With over 20 years of expertise, Jalon delivers customized absorbent solutions across a comprehensive product line, from drying to separation. Our PX adsorbents leverage specialized channel structures, selectively capturing p-xylene based on molecular affinity while ensuring seamless separation from other xylene isomers. They have high adsorption capacity, good compatibility with feed, and strong mechanical stability, which are ideal for p-xylene separation in advanced processes.
Jalon’s dedication to innovation and quality is reflected in 112 patents, ISO 9001 & 14001 certifications, and operations in over 86 countries for dependable performance. Through constant research and development and technical service, Jalon enhances your p-xylene production to achieve higher output and stable operation. To learn more about how Jalon can unlock your process potential, please contact us.
Applications of p-Xylene: From PTA to Advanced Materials
p-Xylene’s true function is achieved in its conversion into a wide spectrum of derivative products that impact almost all aspects of the contemporary world. Here is it’s main and most crucial use:
| Key Uses | Main Application Areas |
| Raw material for PTA (Purified Terephthalic Acid) | – Production of PET (Polyethylene Terephthalate) |
| Primary component of PET (Polyester) | – Beverage bottles, food packaging, plastic films (transparent, durable, recyclable) |
| Production of polyester (PET fibers) | – Clothing, home textiles, industrial fabrics (wrinkle-resistant, durable, versatile) |
| DMT (Dimethyl Terephthalate) | – Alternative monomer for polyester production |
| PIA (Isophthalic Acid) | – PET resin modifier (enhances durability and performance) |
| High-performance specialty polymers | – Engineering plastics such as PBT (Polybutylene Terephthalate) |
| Solvents and chemicals | – Paints, coatings, adhesives, inks |
| Agricultural chemicals | – Production of pesticides and fertilizers |
| Fundamental raw material in material science | – From everyday plastic bottles to advanced engineering plastics, shaping modern life |
Market Analysis and Future Trends of the p-Xylene Industry
The p-xylene industry is not a stagnant one, it is a dynamic industry that is influenced by the global economy, the changing consumer needs and wants, and the constant search for innovation. It is important for the stakeholders to understand the current market and its future trends in order to operate in this challenging industry. The p-xylene market is directly related to the demand for PET and this is due to the packaging, textile and beverage industries. The increase in these sectors especially in the developing countries drives the demand for p-xylene. Fluctuations in the crude oil prices, changes in the consumer trends towards the use of sustainable packaging and the economic cycles are some of the factors that affect the p-xylene market.
Several factors are expected to influence the future of p-xylene industry in the following ways. The rising awareness of sustainability on the global platform has led to the development of new pathways for the production of p-xylene from bio-based feedstocks as opposed to the conventional fossil feedstocks. Technologies for recycling PET are also emerging to ensure that p-xylene and its derivatives form a closed-loop economy. The production of p-xylene has been on the rise due to the advancement in technology in the production processes such as catalysts and process integration. Market intelligence, therefore, is not only about monitoring the current prices and volumes. It is about being able to predict these changes and the dynamics of the economic, environmental and technological factors that will shape the future of the p-xylene industry. It is about having a vision, not just the vision of the current state of affairs.
Environmental Considerations and Sustainable Practices in p-Xylene Production
Like any other large-scale industrial process, p-xylene production has its fair share of environmental impacts. Meeting these concerns and adopting sustainable practices is not only the right thing to do; it is becoming the right thing to do for business. The conventional synthesis of p-xylene has been done through fossil-based feedstocks, which are not sustainable due to their negative impacts on the environment. Thus, the industry is actively in search of environmentally friendly solutions.
Sustainable practices in p-xylene production encompass a range of approaches. The use of biomass as feedstock is a viable approach to the reduction of dependence on fossil resources in the production of chemicals. Optimizing the catalyst and integrating the process reduces energy use and emissions in production processes. Waste minimization and utilization of waste products as valuable products are some of the strategies of circular economy. In addition, there are possibilities of reducing the emission of CO2 from the existing p-xylene plants through the use of carbon capture and storage technologies. The process of achieving sustainable p-xylene production is complex and needs innovation in all the stages of the value chain, from feedstock to waste disposal. It is a promise to sustainable chemistry, which means that the positive aspects of p-xylene will be achieved without any harm to the environment.

Technological Advancements and Innovations in p-Xylene Production
The demand for higher yields, selectivity, and sustainability of p-xylene is a never-ending process that stimulates the development of new technologies. The industry is in constant search of such solutions that can revolutionize the production processes and set new standards. Catalyst development is still a key area of emphasis. Scientists are always looking for new structures of molecular sieves, new materials for catalysts, and new methods of preparing catalysts to improve their activity, selectivity, and stability. Techniques that seek to combine several process steps into a single, more efficient unit operation are known as process intensification strategies. Reactor designs such as reactive distillation, membrane reactors, and others are expected to help in cutting capital costs and energy usage.
Other factors are also emerging, such as digitalization and process control. Automated process control, data management, and artificial intelligence are being used in the plants to control the processes and increase efficiency. These are not just evolutionary changes; these are revolutionary changes that are revolutionizing the face of p-xylene production. They reflect the industry’s dedication to progress, to never stop seeking for better, more sustainable, and more efficient methods of making this fundamental chemical component. It is a process of evolution, based on creativity and the need to progress and develop.









