Introduction to Zeolites
Have you ever met materials that can capture and release molecules of interest while excluding all other molecules? If not, let me introduce you to zeolites. Zeolites are microporous, crystalline aluminosilicate minerals that are widely used in various industries due to their unique properties. These fascinating minerals are well known for their remarkable molecular sieving characteristics. In other words, zeolites have the ability to selectively filter molecules depending on their size and shape. The name ‘zeolite’ is derived from the Greek words ‘zein’ meaning to boil and ‘lithos’ meaning stone, which is due to the fact that when heated, the stones release water. But what is the meaning of zeolite apart from this? It is now time to look at the structure of surfactants, the natural and synthetic types, and the uses of surfactants in various industries, including laundry soaps and air fresheners.

Types and Formation of Zeolites
Natural Zeolites
Natural zeolites are mainly located in volcanic and sedimentary rocks. These aluminosilicate minerals are created through the interaction of volcanic glass with alkaline water that takes millions of years. The major sources of natural zeolites are countries such as United States, South Korea, and Japan. Clinoptilolite and mordenite are the two most dominant minerals that are found in the zeolite structure. These natural varieties are used for mining purposes for water purification and as pet litter. Due to their special structures of zeolite, they are extremely suitable for adsorbing water and other polar molecules.
From an environmental point of view, natural zeolites are very effective in the removal of heavy metals and other pollutants and therefore are very useful in waste management. The regularity and constancy of their architectures allow for a great deal of versatility in numerous uses. Therefore, the next time someone asks you, “What is zeolite used for?” you can tell them that they have the natural ability to purify.
Synthetic Zeolites
However, synthetic zeolites are even more remarkable as they are designed to have even more specific characteristics. These are synthesized through hydrothermal reactions using raw materials such as silica and alumina under specific conditions. The International Zeolite Association, among other bodies, is concerned with synthetic zeolites for even more specialized uses such as ion exchange and catalytic uses in the petrochemical industry. Synthetic zeolites can be designed to have certain size of pores and certain ion exchange capacities. This makes them very useful in areas such as laundry detergents where they aid in softening of hard water, thus increasing the effectiveness of soap.
The importance of synthetic zeolites is evident in the global laundry detergent market, which uses these materials extensively. The synthesis conditions can be adjusted to achieve desired properties such as higher ion exchange capacity or thermal stability of the zeolites. This flexibility makes synthetic zeolites a cornerstone in many industries.
Here’s a table summarizing the key differences between natural and synthetic zeolites:
| Category | Natural Zeolites | Synthetic Zeolites |
| Formation Process | Formed through interaction of volcanic glass with alkaline water over millions of years. | Synthesized through hydrothermal reactions under specific conditions. |
| Common Examples | Clinoptilolite, Mordenite | ZSM-5, Zeolite Y |
| Applications | Water purification, Pet litter, Waste management | Laundry detergents, Petrochemical catalysts, Gas separation |
| Properties | ||
| Selective Reactivity* | Can interact selectively with various cations and molecules. | Can be engineered for even higher selectivity towards specific molecules. |
| Crystallinity* | Highly crystalline structure. | Highly crystalline structure, with more tailorable pore size distribution. |
| Thermal Stability | Can withstand high temperatures without decomposing. | |
| Reversible Dehydration | Retains structural integrity during water removal and rehydration. | |
| Low Solubility | Low solubility in water and most solvents. | |
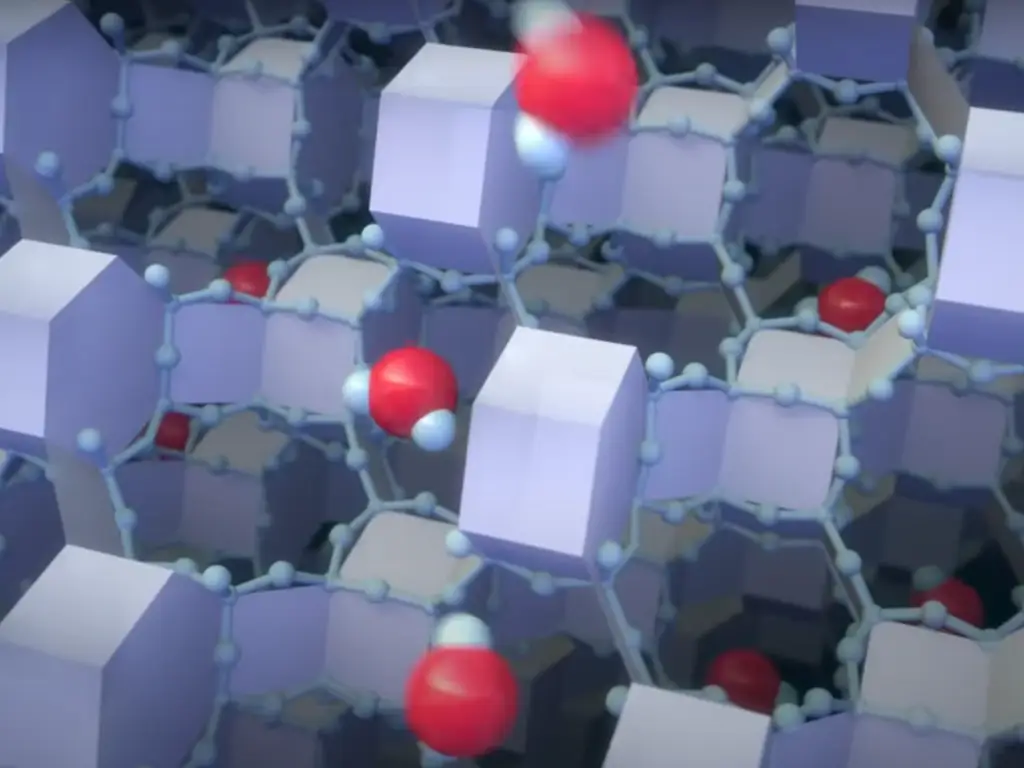
Chemical Composition and Structure of Zeolites
Aluminosilicate Framework
The basic framework structure of zeolites is derived from aluminosilicate materials. Zeolites are mostly composed of silicon, aluminum, and oxygen, which are connected in a tetrahedral manner. These tetrahedra are linked to each other in such a way that they share the oxygen atoms, and the structure is formed in a way that it has a lot of cavities in between the structures. This structure enables zeolites to accommodate cations such as sodium, potassium, and calcium, which can be replaced by other metal ions; thus, zeolites are good cation exchange materials.
Due to the feature of the aluminosilicate framework that can accommodate a variety of cations, zeolites are very useful. These frameworks are accountable for the material’s ability to selectively capture and release ions depending on their size and charge. This functionality is the reason why you may come across zeolites in everything from water softeners to catalysts in industrial processes, utilizing both natural and synthetic zeolites. For instance, the use of synthetic zeolites in industrial processes often relies on the principal raw materials that form their highly effective ion-exchange capabilities, evident in their role as principal raw materials for water softeners and industrial catalysts.
Tetrahedral Structure and Formula
Knowledge of the zeolite formula makes it easier to appreciate the nature of these materials. Every silicon (Si) and aluminum (Al) atom is in the middle of a tetrahedron and bonded to four oxygen (O) atoms. The general formula for zeolites is Mx/n[(AlO2)x(SiO2)y]·zH2O, where M is the cations and n is the valence of these cations. This structure is highly uniform and predictable, and can be used in specific ways, like in the formation of zeolite catalysts.
Furthermore, this tetrahedral structure allows for the addition of other cations and thus the modification of the zeolite for various industrial uses. Whether it is the use of ion exchange in water softening or catalytic activities in the oil and gas industry, the role of the formula in the functionality of the zeolite cannot be overemphasized.
Properties of Zeolites
Zeolites are known to be thermally stable, meaning that they do not break down when exposed to high temperatures, and this makes them suitable for use as catalysts in industrial processes. This stability is due to their rigid structures made of aluminosilicate that do not change even under high temperatures. Therefore, zeolites find application in petrochemical industries and as molecular sieves in many high temperature applications, which makes them important in processes that demand efficient and long lasting filter media. Furthermore, zeolites are characterized by a high degree of crystallinity and stability of the framework during reversible dehydration which is very important for the materials used in the processes which involve water and high temperatures, for example in the catalytic converters and drying industries. These qualities describe the practical benefits of using zeolites in various technologies.
As remarkable as their stability is, zeolites also have low solubility in water and most solvents, which further extends their durability in various applications. Another aspect that can be considered as a major advantage of these complexes is their selective reactivity. Zeolites can selectively interact with various cations and molecules and can be used for various purposes, including filtering out contaminants and as catalysts. This selective interaction is one of the reasons that zeolites are used in water filtration systems where they are able to filter out heavy metals and other contaminants.

Applications of Zeolites
Ion Exchange and Water Softening
The most conventional application of material zeolite is in the ion exchange processes, particularly in water softening. Water that is hard contains calcium and magnesium ions, which can lead to formation of deposits, including soap scum and scale in pipes. The unwanted ions are replaced by sodium ions or other less harmfully charged cations and thus the water is softened. The importance of synthetic zeolites is evident by their application in the global laundry detergent market. In this way, the soft water in the washing machine allows for more effective cleaning, reduced wear of fabrics, and a longer service life of appliances with the help of zeolites. Well, the next time you come across a detergent commercial that promises to soften water, just remember that zeolites are at play!
Zeolites boast important commercial applications owing to their purity and uniformity. They are among the most abundant mineral components utilized in industrial processes and are produced in large quantities, with tonnes of zeolites being employed annually to meet industrial demands.
Catalytic Uses
Zeolites also have their merits in catalytic applications, especially in the petrochemical sector. The structure of the pores is such that certain molecules can penetrate and interact while others cannot, making them useful for catalyzing certain reactions while preventing other undesired side reactions. One of the most familiar uses is in the breaking down of large hydrocarbon molecules into smaller and more useful ones such as gasoline. The regular pore size distribution in zeolites makes it possible to achieve selective catalysis, which improves the efficiency and yield.
Zeolite catalysts have become very important in several industries due to their efficiency in catalysis. Industries have been able to cut on their cost and energy when using the zeolite-based catalytic processes. These catalysts are also used in minimizing emissions from industrial exhausts, thus playing a role in environmental conservation.
Adsorption and Desiccation
Zeolites are good adsorbents which have the ability of attracting and holding water molecules. This makes them suitable for use as desiccants in different industrial activities where it is important to keep the environment dry. For example, in the pharmaceutical industry, zeolites are employed to maintain the moisture content of packaging materials, ensuring that products sensitive to moisture do not expire before their time. The fact that they do not degrade their structure when they are subjected to reversible dehydration makes it possible for them to perform optimally in successive drying processes.
Apart from industrial desiccation, there are other uses of zeolites in air purification and gas separation. They can selectively adsorb specific gases, for instance carbon dioxide or ammonia, and are therefore very useful in environmental remediation. In air purification systems, zeolites are used to capture toxic substances and make the air in homes and industries safe for use. The ability to adsorb and selectively separate gases is also used in medical applications like oxygen concentrators used in respiratory therapies.

Environmental and Commercial Uses
Zeolites are widely used in waste treatment and environmental remediation processes. Due to their selective adsorption characteristics, they can selectively remove and immobilize pollutants, heavy metals, and other toxicants from wastewater. This makes them useful in such areas as mining and smelting where the wastewater is likely to contain toxic metals. Zeolites can be applied in environmental cleanup activities where soils and groundwater are polluted. They are capable of selectively adsorbing dangerous ions and molecules that are useful in the process of decontaminating the areas that have been affected by the pollutants, thus making them suitable for habitation and farming. This makes zeolites an important factor in attaining sustainable environmental management.
Apart from the industrial uses, zeolites also have uses in our daily lives. One of the most well-known applications is in the pet industry, where it is used as a major component of cat litter. Due to the high absorption capacity of zeolites, they are ideal for use in pet litter as they help in controlling odors and moisture, thus creating a cleaner environment for pets. Also, zeolites are applied in household water filters, where ion exchange is useful in water softening and purification.
Health and Safety Considerations
However, it is important to look at the health and safety effects of zeolites despite the numerous advantages they come with. In general, zeolites are not toxic and can be used in most cases; nevertheless, the dust of these materials is dangerous when inhaled. One should avoid inhaling the dust produced by zeolites and therefore it is recommended that zeolites should be handled in places with good ventilation or one should wear protective gear such as a mask. Furthermore, it is also important to note that inhalation of zeolite dust may lead to skin irritation, so it is advisable to wear gloves when handling the material. The proper storage and disposal should also be observed so as not to negate the positive impacts of zeolites on the environment due to health complications.
Conclusion
To sum up, zeolites are universal minerals that can be used for water softening, air purification, catalytic processes, and waste treatment. Due to their characteristics, they are indispensable in various industries and households. The knowledge of the meaning of zeolite is not limited to the question “what is zeolite” but also entails recognizing the great impact that they have made in the current society. From the formation of natural zeolites in volcanic rocks to the synthesis of different types of zeolites, they are still transforming industries and enhancing the environmental impact.






